TakeMeHome™ – a free HIV/STI self-testing program
In partnership with Emory University, OraSure Technologies, NASTAD, and Signal, Together TakeMeHome makes HIV self-testing a reality nationwide. Based off of the TakeMeHome™ self-testing model, Together TakeMeHome expands access to HIV self-testing throughout the U.S., including Puerto Rico, for everyone ages 17 and older. TakeMeHome™ continues to offer an expanded set of services, including STI testing, PrEP labs, and rapid in-home syphilis tests in participating areas. Want to sign up? Read more below or view our flyer.
TakeMeHome™ continues to offer an expanded set of services, including STI testing, PrEP labs, and rapid in-home syphilis tests in participating areas. Want to sign up? Read more below or view our flyer.
Building Healthy Online Communities (BHOC), in partnership with Emory University and NASTAD, developed the National Home Test Kits program for state and local health departments to offer confidential HIV and comprehensive STI testing delivered securely and discreetly directly to their constituents. TakeMeHome™ is designed with a focus on reaching men who have sex with men (MSM) and trans/non-binary people who use dating apps, though the platform is open to all people over age 17* who haven’t had a recent HIV or STI test. We are eager to support health partners who are also prioritizing other communities who could benefit from self-testing, including cisgender women of color, disabled people, sex workers, unhoused people, and people who inject drugs. This service is currently offered in English and Spanish. *Age of participation varies based on local laws and program partner eligibility criteria.
Why we support self-testing:
- Self-testing is a great way to reach those who aren’t getting tested. Fully 1/3 of TakeMeHome™ users report never having had an HIV or STI test before. Additionally, the 2019 American Men’s Internet Survey found that 22% of MSM who use dating apps reported that they had NEVER tested for HIV.
- Research on HIV and STI prevention strategies supports self-testing. Check out a recent paper here.
- It’s endorsed by people who face significant barriers to on-site testing services. TakeMeHome™ users–especially those who are trans, disabled, or living in rural areas–have told us that self-testing is the best option to keep them on top of their health.
Updates (as of April 5, 2025):
- TakeMeHome’s team has partnered with Grindr to make it possible in select international locations to order a self-test on the dating app. Read more at ThinkGlobalHealth.
- TakeMeHome is the first organization in the U.S. to offer in-home rapid syphilis testing. Read the announcement here.
- The U.S. Food and Drug Administration recently expanded the age range for OraQuick in-home HIV tests to ages 14 and up. TakeMeHome is working with our partners to expand eligibility in our program in the coming weeks.
- The June 2024 CDC’s Morbidity and Mortality Weekly Report highlighted our program.
- Follow us on Instagram & Tiktok for program updates.
Check out the ordering process for STI test kits and HIV oral swab tests below.

“What a great program. Smooth process without the anxiety of a doctor’s office.”

“After losing my job and benefits, I could not afford the tests any longer. Thank you for this wonderful service.”

“Wonderful service as I’ve been too scared to go get tested. This’ll make it much easier for me just to take at home. Otherwise, I’m not sure when I would get the courage to go get tested.”
Program Background
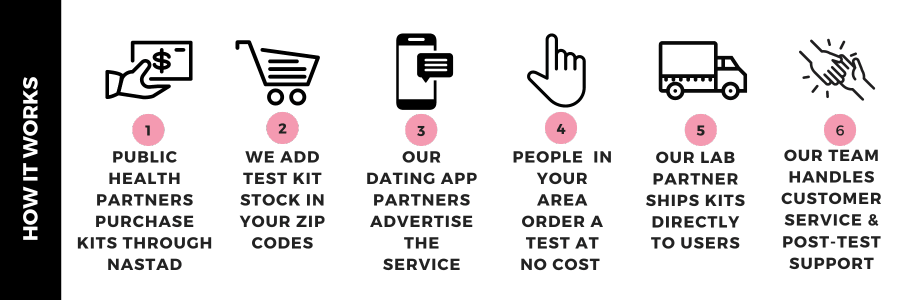
To make mailed HIV/STI testing most efficient for public health partners, we created the following strategy:
1) Centralized system for HIV/STI test kit ordering and distribution
We have developed a centralized HIV/STI test kit-ordering and fulfillment system. Public health agencies who opt in to using the system can choose the type and numbers of mailed tests, the duration of their participation, and the eligible populations they would like to reach.
2) Testing options
There are two different types of testing packages that can be set up:
HIV Oral Swab (OraQuick®) Testing
Users will be sent an HIV oral rapid in-home test, with detailed instructions and information about confirmatory testing and HIV and STI prevention and care resources.
Rapid In-home Syphilis (First to Know®) Testing
As of December 2024, our program is only offering rapid in-home syphilis test as an add-on to an HIV Oral Swab order. Users will be asked about their syphilis history and their interest in receiving the test (if eligible). The self-test will be sent with their HIV Oral Swab test, with information on how to use the test and what to do after receiving results.
Lab-based Testing
STI testing 3-site collection and dried blood spot testing is available in select areas, which users can send specimens to a laboratory in the postage-paid return packet that will be included. Syphilis and Hepatitis C testing are available for those who are eligible. Pending a decision by the U.S. FDA, we are currently not able to offer HIV blood testing and are sending HIV Oral Swab Tests in its place. Due to this change, please contact us to confirm the availability of HIV blood testing, including PrEP labs.
3) Promotion
Dating apps promote this opportunity through messaging and advertisements. New jurisdictions will get a minimum of 2 messages per month for the first 4 months of participation. Users of geospatial social networking apps, like Grindr, can order by clicking on a link embedded within sexual health-related content of their apps. Public health agencies can also include the ordering links in their other health promotion materials. We provide a bilingual (English/Spanish) media toolkit of images with captions for use in digital promotion and printed outreach materials.
4) Results delivery
HIV Oral Swab (OraQuick®)
Users of the OraQuick® self-administered swab will get their results at home. After their test, participants can self-report the result of their test in our follow-up survey.
Rapid In-home Syphilis (First to Know®) Testing
Users of the First to Know® self-administered blood test will get their results at home. After their test, participants can self-report the result of their test in our follow-up survey.
Lab-based testing
We also offer laboratory-based STI testing. In locations where STI testing is available and users select it, participants are able to access the results of self-collected specimens after lab analysis using a secure results portal. Users with positive test results will get follow-up through their local jurisdiction, as well as links to geo-targeted testing, treatment, prevention, and other healthcare services. All users will receive basic information about STI testing, PrEP, doxyPEP, condoms, U=U, harm reduction, and pregnancy. All positive results for tests with required reporting will be automatically reported to the health department by our lab partner, Molecular Testing Labs.
5) Costs of participation:
The cost is calculated based on the number of tests purchased. This cost includes promotion through the apps (though jurisdictions may also choose to supplement this with their own advertising efforts), test kits, delivery/fulfillment, and participant survey data.
For specific pricing information, please contact Jen.
Frequently Asked Questions
Has this been tried before? What’s the evidence that it works?
Virginia, Arizona, and New York City have piloted the delivery of self-test kits and have found that this process enabled them to reach individuals who hadn’t tested recently. In most cases, they have had a higher positivity rate than traditional testing strategies.
- New York City: 28% of testers hadn’t tested in the previous year and 14% hadn’t ever tested. They reported a positivity rate of 0.3%
- Virginia: 29% of testers hadn’t tested in the previous year and 21% hadn’t ever tested. They reported a positivity rate of 1.3%; 88% of new positives were linked to care within 30 days.
- Arizona reported a positivity rate of 1.2%.
In 2021 and 2024, CDC highlighted TakeMeHome as a highly impactful model, especially during the ongoing COVID pandemic.
How is this program run? Who houses the testing kits and fulfills orders?
TakeMeHome and its delivery subcontractor takes care of all that. All of these services are included in the cost. Each health department orders the number of kits they want; TakeMeHome takes care of the rest, including promotion. Additionally, health departments may modify the numbers of tests they order during a given time period.
Who are these tests trying to reach?
Promotion will happen predominantly through dating apps that cater to gay, bi, and trans people due to our relationships with these apps. Health departments have the opportunity to promote this program to whichever populations they choose and will have greater flexibility to choose their eligibility criteria. We are looking to expand our reach with other vulnerable populations as the program evolves.
What happens to individuals from non-participating jurisdictions?
They are given information about other self-testing options or testing sites near them, through the CDC-maintained database.
What happens to those who are not eligible?
If a user is ineligible, either because they do not live in a participating jurisdiction or because they have been tested within the past 3 months, TakeMeHome will link them to other self-testing or test sites near them. The site also has information regarding prevention strategies.
Is this service available in Spanish?
Yes! From start to finish, participants can order in Spanish. All of TakeMeHome’s materials are bilingual to help create language access for speakers of English and Spanish. Additionally, we partner with some dating apps to send Spanish language promotional messages about self-testing.
Can PS18-1802 HIV grant funding, EHE, or other funds be used to participate in the program?
You can use PS18-1802 funds, EHE funds, or other funds for this program. Payment is made through NASTAD.
What’s the process and options for distributing State-specific messages or information about State/local resources in association with the test kits?
Our platform is built with that in mind. Through our partnerships with dating apps, we can reach people specifically in your jurisdiction Each participating jurisdiction compiles local resources that are available to users in their area.
Can an individual order more than one HIV oral swab test?
Yes, jurisdictions could opt between allowing participants to order just one HIV oral swab test for themselves or two. In the future, we are open to expanding this more widely, based on requests from jurisdictions.
Evidence has pointed to increased positivity rates among individuals who give test kits to their partners and peers.
How will our surveillance team know about new positive results for HIV oral swab tests?
It’s helpful to remember that at any point, anyone in your jurisdiction can walk into a Walgreens and purchase this test over the counter or order one online. The only difference is that your state is now supporting people who haven’t tested recently to get easier access to these tests. Your surveillance team will know about these positives as individuals come in for confirmatory testing or if individuals chose to share their results through the follow-up survey. We encourage each participating jurisdiction to run periodic matches between the data we send and their surveillance data.
Which tests are included in STI testing?
STI testing includes 3-site chlamydia and gonorrhea, syphilis, and HIV. HCV testing is included based on the user response to a risk questionnaire.
How does syphilis testing work?
For at-home collection of syphilis samples, our lab partner will provide a dried blood spot (DBS) card.
The DBS sample is first tested using an EIA assay for the qualitative detection of IgG antibodies to T. pallidum.
- If negative, then the sample is reported as “not detected.”
- If positive, then the sample is repeated in duplicate using the EIA assay.
The client is informed through the results portal that they need to get additional testing and follow-up care.
Only clients who have not had a previous syphilis diagnosis will receive syphilis testing. Those who self-reported testing positive for syphilis before will be informed they will not receive syphilis testing and linked out to local resources.
What happens with linkage to care and support for individuals who test positive?
HIV Oral Swab (OraQuick)
For OraQuick tests, individuals will get information in their test kits about what to do in the case of a positive result. This will include how to obtain a confirmatory test at a local clinic and the number of OraSure’s hotline if they need support. (Some states or jurisdictions may already have or want to create a hotline for this purpose.)
Following a preliminary positive result at home, when an individual comes in for confirmatory testing, they will be known to the jurisdiction’s HIV care and surveillance systems.
Lab-based testing
For lab-based testing, users can access their results through a client portal. The health department will also receive all results through a care portal and positive results through a secure data submission process from our lab partner, Molecular Testing Labs.
What data will be made available to participating health departments?
We will make the following data available:
- # of people from each jurisdiction who visited to the TakeMeHome website
- # of site visits and order conversion rates
- Client-level detail for each kits sent out, including: Name, Mailing address (including zip code), Email, number of kits ordered, Age, Sex at Birth, Time since last HIV or STI test (phone number required for lab testing)
- Optional additional order information includes: race/ethnicity, gender identity, location of last HIV test, number of sex partners in past year, current PrEP use, health insurance status
- # of kits sent out
- Follow-up client surveys include the following: Race/ethnicity, Gender identity, Transmission category, Where their most recent HIV test was, Whether they took the test, Test result information, Linkage information, Access to PrEP, Access to STI testing, Feedback about their experience
What is in place in regards to data security and HIPAA protections?
We have worked closely with our Compliance Officer, IT, and legal teams to ensure the highest quality data security and HIPAA practices. Our lab testing partner, Molecular Testing Labs (MTL), has a secure lab portal for STI and lab-processed results and they ensure HIPAA protections are in place for all of their services. We have a signed BAA in place with MTL that covers all participating jurisdictions. Each participating jurisdiction can also find a detailed Business Associate Agreement that refers to our relationship with Health Departments here.
What areas are currently participating?
We are excited to be working with our public health partners to expand self-testing across the U.S. Here’s who is on board:
- Arkansas
- California
- Hawai’i
- Idaho
- Indiana
- Kentucky
- Maine
- Michigan: Allegan and Ottawa Counties
- Missouri: St. Louis County, St. Charles County, Franklin County, Jefferson County, Lincoln County, Warren County, Kansas City
- Montana
- New Jersey
- New Mexico
- North Carolina
- Oregon
- Texas: Erath County, Hood County, Johnson County, Palo Pinto County, Parker County, Somervell County, Tarrant County, Wise County
- Wyoming
List updated April 12, 2025
Contact jenhecht@bhocpartners.org to learn more
Additional Information
TakeMeHome Research & Presentations
- How Dating Apps Could Unlock At-Home HIV Testing (ThinkGlobalHealth, April 2025)
- New HIV infections fall sharply in SF after years of slowing progress (San Francisco Chronicle, September 2024)
- TakeMeHome: A novel method for reaching previously untested people through online ordering and self-collect HIV and STI testing (Sexually Transmitted Diseases, 2024)
- Findings from the First Year of a Federally Funded, Direct-to-Consumer HIV Self-Test Distribution Program — United States, March 2023–March 2024 (Centers for Disease Control and Prevention, 2024)
- TakeMeHome: How BHOC and Grindr partner to distribute HIV self-tests, (U.S. Department of Health and Human Services Briefing, 2023)
- Issue Brief: The Role of HIV Self-Testing in Ending the HIV Epidemic (Centers for Disease Control and Prevention, 2022)
- Increasing Access to HIV Testing Through Direct-to-Consumer HIV Self-Test Distribution (Centers for Disease Control and Prevention, 2021)
- Self-Testing Strategies for HIV Testing and PrEP Access: Providing At-Home Testing Through the BHOC Partnership (NASTAD, 2020) | Webinar Video & Slides
- Rapid Uptake of Home-Based HIV Self-testing During Social Distancing for SARS-CoV2 Infection in Oregon. (Journal of AIDS and Behavior, 2021)
- National Home Test Program Project Brief
Self-Testing Resources
- Greater Than AIDS has launched a webpage of FREE HIV self-testing programs available around the country to help individuals find more options during COVID-19.
- CDC has self-testing resources through their Let’s Stop HIV together campaign.





